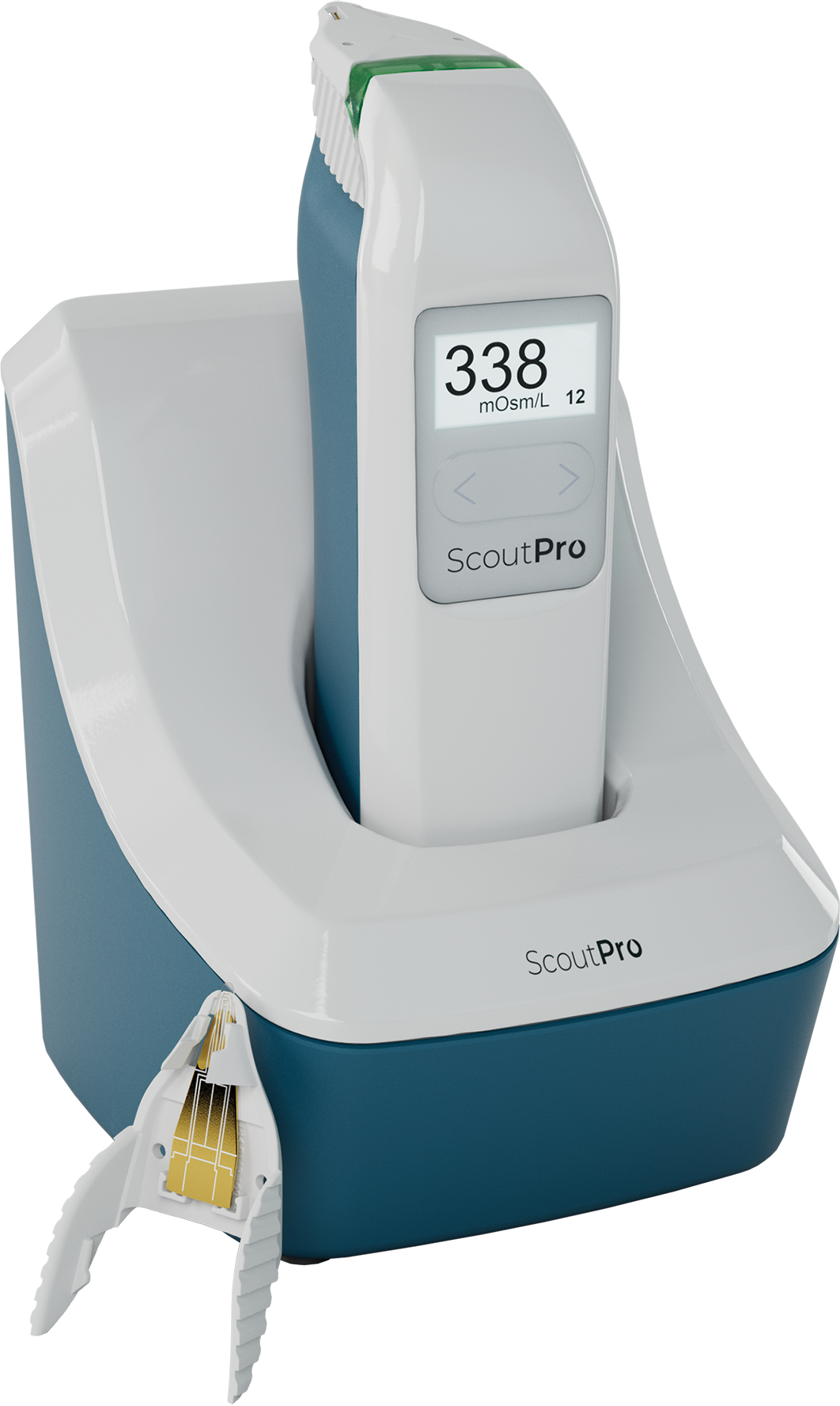
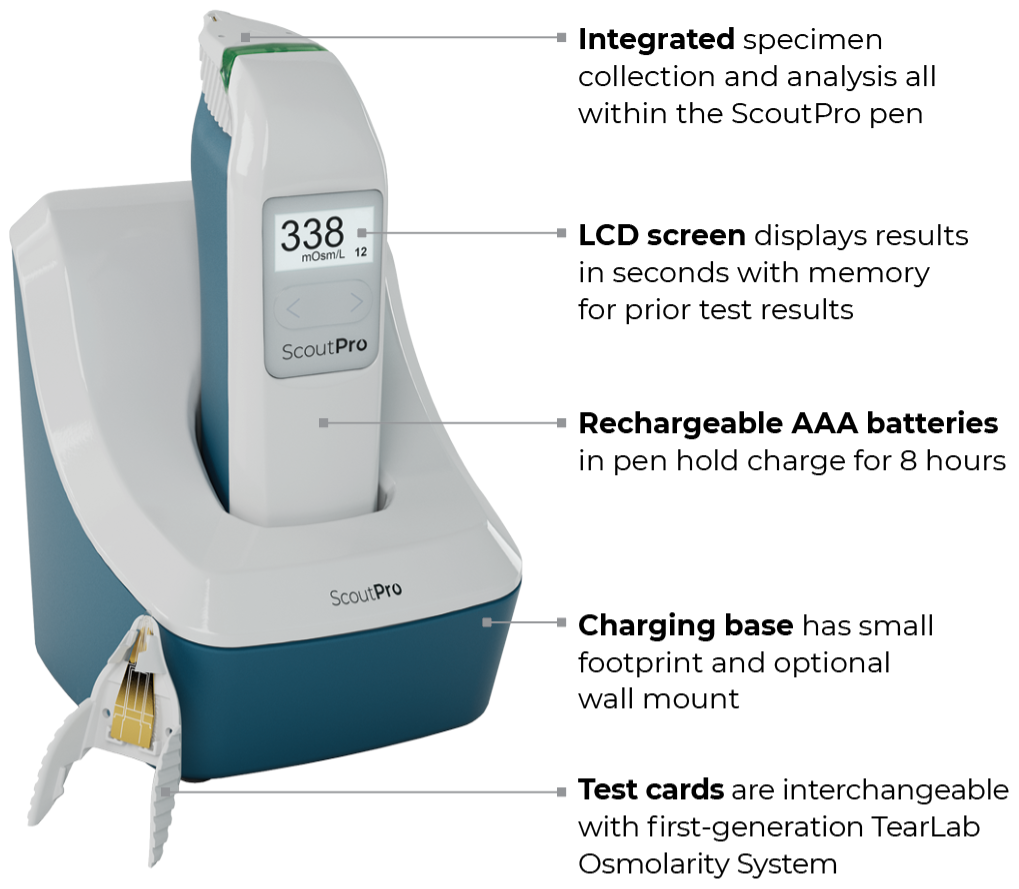
Portable
ScoutPro Osmolarity System
Toxic hyperosmolarity can damage corneal cells and drive refractive instability1-3

Unmask a hidden cause of 20/20 unhappy patients.
Learn how tear osmolarity may impact outcomes and satisfaction following cataract surgery.4
The power of osmolarity testing in the palm of your hand
Indications and Important Safety Information for ScoutPro Osmolarity System
Indications: The ScoutPro Osmolarity System is an automated device intended to quantitatively measure the osmolarity of human tears to aid in the diagnosis of dry eye disease, in patients suspected of having dry eye disease in conjunction with other methods of clinical evaluation.
Contraindications: Do not collect tear fluid from a patient within two hours of medicinal eye drop use or use of topical medications. Do not collect or store tear fluid samples for transport or testing at a later time. Do not collect tear fluid after ocular surface staining. Do not collect tear fluid within 15 minutes of use of anesthetic or mydriatic (dilating) eye drops or after other invasive ocular diagnostic testing. Do not collect tear fluid within 15 minutes after a slit lamp examination. Do not collect tear fluid within 15 minutes from a patient who has been crying.
The ScoutPro Osmolarity System (ScoutPro) is a CLIA Waived test system for human tears. Each laboratory or testing site using the ScoutPro must have a CLIA Certificate of Waiver before starting testing.
The ScoutPro is designed for stability, reliability, and safety, and it has been developed, manufactured, and marketed under a quality management system certified to ISO 13485 (2012).
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
ATTENTION: This is not all you need to know. Please refer to the User Manual for a complete listing of indications, contraindications, precautions, and use information.
References
- Sullivan BD, et al. Clin Ophthalmol. 2024;Aug 28;18:2419-2426.
- Huet E, et al. Am J Pathol. 2011;179(3):1278-1286.
- Epitropoulos AT, et al. J Cataract Refract Surg. 2015;41(8):1672-1677.
- Kursite A, Laganovska G.
J Ophthalmol (Ukraine). 2023;2(511):11-15.